Mole Number
Mole Number
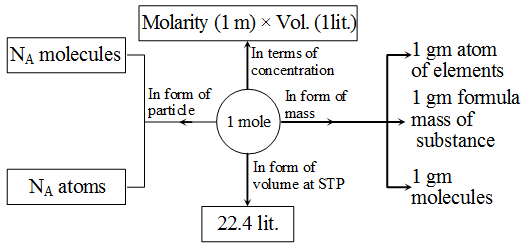
1 mole of atoms = 6.022 × 1023 atoms = Gram atomic mass or Molar mass of element
Avogadro's number
Atoms and molecules are so small in size that they cannot be counted individually. The chemists use the unit mole for counting atoms, molecules or ions. It is represented by n.
A mole represents 6.022 × 1023 particles.
Atoms and molecules are so small in size that they cannot be counted individually. The chemists use the unit mole for counting atoms, molecules or ions. It is represented by n.
A mole represents 6.022 × 1023 particles.

No comments:
Post a Comment